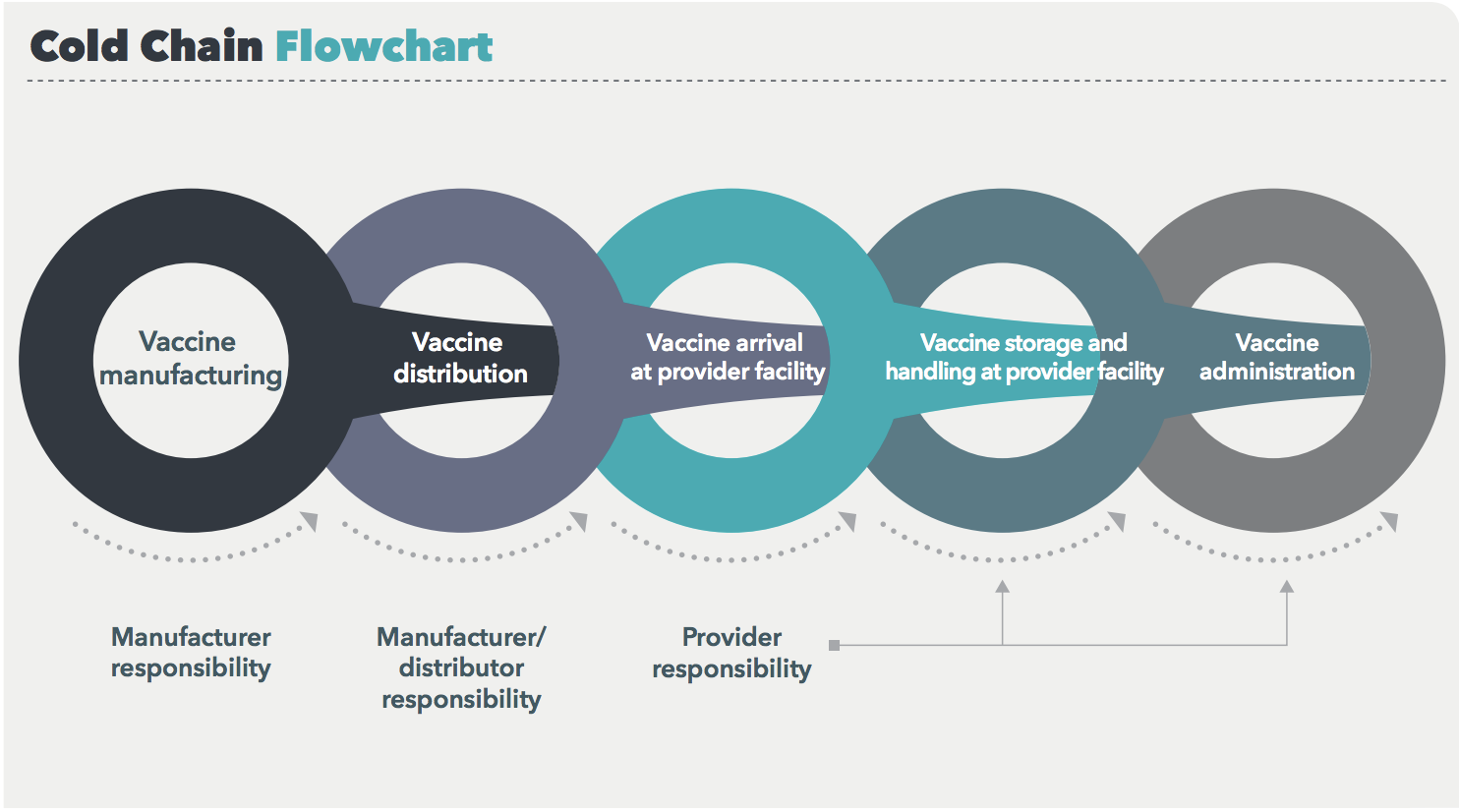
The vaccine cold chain is a complex system with many moving parts and multiple levels of accountability, including manufacturers, distributors, and providers.
Every link in this chain is vulnerable to improper temperatures, which can render vaccines ineffective.
Most of the information about the cold chain and how to improve it focuses on what happens after a vaccine shipment leaves the manufacturing facility. But improper temperature exposure can, and does, occur during the manufacturing process as well, and these exposures can have ripple effects down the line. Here are four reasons for vaccine makers to up their cold chain game.
1. When vaccines don’t work, it puts public health at risk and decreases public trust.
Vaccination is one of the greatest public health achievements in history. In terms of preventing infectious diseases, the only thing more effective than vaccination is clean water.
And yet, the past few decades have seen growth in anti-vaccine attitudes. Globally, the issue has gotten so bad that the World Health Organization named “vaccine hesitancy” one of the top 10 threats to global health for 2019.
Of course, the reasons people choose to forgo vaccines are complex, but for the most part what they boil down to is a lack of trust. When vaccines don’t work, not only are people put at risk, but their trust level sinks even further. To prevent this, stakeholders at every phase of the cold chain need to do everything possible to ensure the effectiveness of these crucial medicines.
2. The effects of improper temperature exposure are cumulative and irreversible.
From the latest version (January 2019) of the CDC’s Vaccine Storage and Handling Toolkit [emphasis added]:
Vaccines must be stored properly from the time they are manufactured until they are administered. Potency is reduced every time a vaccine is exposed to an improper condition. This includes overexposure to heat, cold, or light at any step in the cold chain. Once lost, potency cannot be restored.
Vaccine manufacturers are responsible for the very first link in the cold chain. By doing everything they can to ensure that vaccines leave their facility at full potency, drugmakers can boost the chances that the final product administered to the patient will still be potent. In contrast, if a vaccine’s potency is diminished before it even leaves the manufacturing facility, the likelihood of an ineffective vaccine reaching a patient increases.
3. The first link in the cold chain is the easiest to control.
As we mentioned above, every link in the cold chain is vulnerable. But they aren’t equally easy to control.
For example, picture a vaccine that’s manufactured in the United States and transported to a developing country. Even if that vaccine managed to get from the manufacturer, onto an airplane, to a central store, and to a regional store without being exposed to improper temperatures, it still might have to travel up a mountain via backpack to a local health clinic.
That’s an extreme example, but vaccines are often administered in areas where the electrical system is unreliable or non-existent, making improper temperature exposure a common occurrence. That’s why the first link in the chain is so important.
4. There’s a common — and unrecognized — break in the cold chain during the manufacturing process.
If you’re a vaccine manufacturer, you might be thinking, “I know all of this. But what can we do? We already hold our vaccines at the correct temperature from the minute we manufacture them until the minute they head out the door.”
But is that true?
In many traditional vaccine manufacturing processes, there’s a common break in the cold chain that’s only recently come onto the radar.
Typically, vaccines are manufactured and then held in a refrigerator or freezer until they’re ready to be sent to a customer. When the vials are taken out of cold storage, condensation forms, which needs to dissipate before inspection and labeling can occur. So, the vials are put into a conditioning room and allowed to dry for 24 to 48 hours. Once they’re dry, the vials are inspected, labeled, and shipped.
Because of the need for the vials to dry, vaccines are routinely kept out of the cold chain for a day or two before they leave the manufacturing facility. During that time, their potency can start to decrease. If the drugs are exposed to additional improper temperatures down the line, they may no longer work by the time they’re administered.
To tackle the problem, we developed an Automatic Vial Dryer that can dry vials in as little as 5 minutes, significantly reducing the time vaccines must spend out of the cold chain. Check out our recent press release to learn more about the system and watch a video of it in action.
We’ve been partnering with pharmaceutical companies like yours for decades to help improve processing lines. Visit our Pharmaceutical Solutions webpage or contact us to learn more about how our equipment can benefit you.
Image source: CDC